Experiment 1: THE EFFECT OF DIFFERENT AMOUNT OF PEG
ON THE PHYSICAL CHARACTERISTICS OF SUPPOSITORY
Title:
The effect of different amount of PEG on the
physical characteristics of suppository.
Objectives:
1.
To calibrate
suppository mould with PEG before preparing medicated suppositories.
2.
To determine
the effect of different compositions of PEG base on the physical
characteristics of suppositories.
Date
of experiment:
7th March 2017
Apparatus
and Materials:
Analytical
balance, 1 x suppository mould set, water bath at 37o C ,1 x
spatula, hotplate, 4 x weighing boats, 4 x 50 mL beaker, 2 x glass rod, 1 x 5
mL pipette and pipette bulb, 1 x 5 mL measuring cylinder, polyethylene glycol
(PEG)1000, distilled water, polyethylene glycol (PEG) 6000, liquid paraffin,
and paracetamol.
Introduction:
Suppositories
are solid dosage forms of various sizes, appearance (shapes) and weights
intended for administration by rectal route where they melt, soften or dissolve
to exert their effect. They are capable of being easily inserted into the
intended orifice without causing undue distention. The suppository usually
composed of a medicament incorporated (dissolved or suspended) in a suppository
base, this medicament may be intended for retention within the cavity for
localized drug effect or to be absorbed for the exertion of systemic effect.
For example, rectal localized action such as relief of constipation, pain,
itching and inflammation associated with hemorrhoid conditions.
Suppositories
are indicated for systemic action in pediatric patients and in patients who
cannot take or tolerate oral medication due to variety of reasons e.g. to
relief nausea, vomiting and pain. The drug must be spread in a suitable base of
suppository. Ideal suppository bases should be easily formed by compression or
molding; release any medicament readily; melt at body temperature or dissolve
or disperse in body fluids; keep its shape when handled; compatible with the
drugs, non-irritant and non-toxic.
Polyethylene
glycol (PEG) polymers have received much attention as suppository bases in
recent years because they possess many desirable properties. They are
chemically stable, non-irritating, miscible with water and mucous secretions,
and can be formulated, either by molding or compression, in a wide range of
hardness and melting point. Moreover, they do not melt at body temperature, but
dissolve to provide a prolonged release. Certain PEG polymers may be used
singly as suppository bases but, more commonly, formulas call for compounds of
two or more molecular weights mixed in various proportions as needed to yield a
finished product of satisfactory hardness and dissolution time.
Methodology:
A.
Calibration of Suppository Molds with PEG Base.
For
this calibration exercise, 10g of the following proportions of PEG 1000 and PEG
6000 are used.
Ingredients
|
Percentage
|
Weight Basis
|
PEG 1000
|
60%
|
6g
|
PEG 6000
|
40%
|
4g
|
To
calibrate the mold with PEG suppository base:
1.
Clean
and dry mold was used. The mold was not lubricated.
2.
PEG
1000 was melted on a steam bath or hot plate. Then, the heat was reduced and
mixed in the other PEG.
3.
The
mixture was removed from the heat and it was allowed to cool before pouring
into the mold.
4.
The
cavities of the mold were overfilled with the mixture of the PEG 1000 and PEG
6000. It was left at room temperature until it become solid.
5.
The
excess was removed with a hot spatula. Then, the suppositories were removed
from the mold.
6.
The
suppositories were weight and the total weight was recorded. The average weight
of the suppository was calculated.
B.
Preparation of paracetamol suppositories.
1.
Saturated
stock solution of paracetamol was prepared by adding 10g of paracetamol in 5 mL
of distilled water.
2.
The
paracetamol suppository (10g) was prepared by using the following formulation:
Suppository
|
PEG 1000 (g)
|
PEG 6000 (g)
|
Paracetamol stock solution (mL)
|
Total (g)
|
I
|
9
|
0
|
1
|
10
|
II
|
6
|
3
|
1
|
10
|
III
|
0
|
9
|
1
|
10
|
3.
One
type of PEG was melted on a steam bath or hot plate. Then, the heat was reduced
and being mixed in the other PEG.
4.
The
mixture was removed from the heat and it was allowed to cool before pouring
into the mold.
5.
The
cavities of the mold were overfilled with the mixture of PEG 1000 and PEG 6000.
It was left at room temperature until it become solid.
6.
The
excess was removed with a hot spatula. Then, the suppositories were removed
from the mold.
7.
The
shape, texture and color of the suppositories were observed.
8.
Each
of the suppositories was put into a separate beaker containing distilled water
(10 mL and pre-warmed at 37o C). Then, the beaker was put into a water bath (37
o C).
9.
The time for
the suppositories to melt was recorded.
Results
10g of the
following proportions of PEG 1000 and PEG 6000 were used for calibration
exercise.
Ingredients
|
Percentage
|
Weight Basis
|
PEG
1000
|
60%
|
6.0g
|
PEG
6000
|
40%
|
4.0g
|
Part 3.3.1
No of mold
|
1
|
Total weight for 6 suppositories (g)
|
6.0708
|
Average weight for 1 suppositories (g)
|
6.0708/6 = 1.0118
|
Part 3.3.2
Suppository
|
Shape
|
Texture
|
Colour
|
I
|
Bullet
|
Soft, greasy
|
Clear white
|
II
|
Bullet
|
Hard. smooth, slightly greasy
|
Cloudy white
|
III
|
Bullet
|
Hard, smooth, less greasy
|
White
|
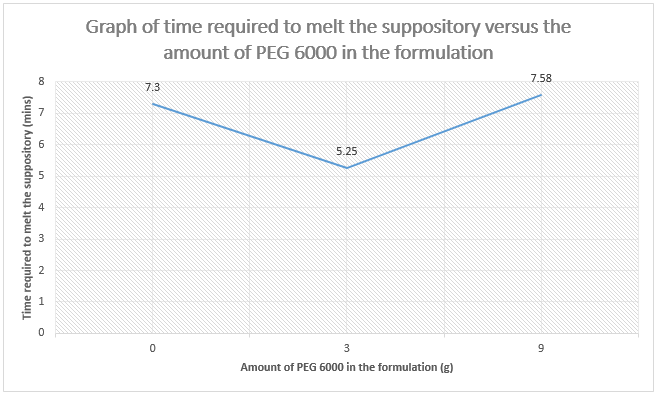
Amount of PEG 6000 (g)
|
0
|
3
|
9
|
Time (mins)
|
7.30
|
5.25
|
7.58
|
DISCUSSION
1.
Describe the important of calibrating suppository mould before preparing
medicated suppository.
Each individual mold is capable of
holding a specific volume of material in each of its openings. The difference
in the densities of the materials will produce suppositories with different
weights. Any added medicinal material will affect more the weight of both bases
and amount of suppository that are able to be put into the mold. Calibrating
suppository mould before preparing medicated suppository is to make sure that
the mould produce an equal size and weight for each of the suppositories. It is
to ensure accurate dosing.
2. Compare the physical appearance
of suppositories that are formed and discuss.
Suppository
|
Shape
|
Texture
|
Colour
|
I
|
Bullet
|
Soft, greasy
|
Clear white
|
II
|
Bullet
|
Hard. smooth, slightly greasy
|
Cloudy white
|
III
|
Bullet
|
Hard, smooth, less greasy
|
White
|
The table above refers to the comparison
of the physical appearance of suppositories with different compositions of PEG
base that are formed during the experiment. The two main bases involved are PEG
1000 and PEG 6000. These PEGs are blended together to produce several
suppository bases with different melting point and physical
characteristics.
All of the suppositories possess the same shape which is bullet shape,
due to the shape of the mould used for
the making of the suppositories.
The melting point of PEG increases with
the rise of the molecular weight of PEG. So, suppository III is the hardest
among all of the suppositories due to the high amount of PEG 6000. Suppository
I possess a high amount of PEG 1000 which explain the soft, gel-like texture.
Suppository III has a flaky texture which
is different from the other two suppositories which also due to the high amount
of PEG 6000. High molecular weight PEG such as PEG 6000 has a high melting point which leads to harder,
brittle and flaky form of suppository formed. As the molecular weight of the
PEG increase, the hygroscopicity decreases. This result in suppository III
showing the least greasiness appearance compared to suppository I and II.
All of the suppositories are in white colour due to the even dispersion of paracetamol
powder in the suppository base. Nonetheless, the suppositories possess a different
intensity of cloudiness. Suppository III has a high
amount of PEG 6000, hence giving it wax-like, white color solid.
3.
Plot a graph of time required to melt the suppository vs. the amount of PEG
6000 in the formulation. Compare and explain the results.
The objective of this
experiment is to determine the time taken for the paracetamol suppository to
melt at the mimic of constant body temperature (37°C ) depends on the
concentration of Polyethylene glycols 6000 (PEG 6000) contained in the suppository. PEG is
generally used as base for suppository due to it is water soluble, inert,
non-ionic and most polar organic solvent. Different molecular weight of PEG will result
in various physical parameters of the products such as solubility, melting
point, freezing point and surface tension.
Theoretically, the molecular weight of PEG is directly proportional to
the melting point of the suppository. The higher the molecular weight of PEG,
the higher the melting point of the suppository. Hence, PEG 6000 has higher
melting point compared with PEG 1000 as their average molecular weight are
between 5000-7000 and 950-1050 respectively. From the graph above, we can conclude
that the amount of PEG 6000 also influences the melting point of the
suppository. As the concentration of PEG 6000 increases, the amount of energy
needed to overcome the attraction force between the paracetamol and PEG
particles increases, therefore more time is required to breakdown the bonds
between the molecules of the compound.
However, the result
that we obtained from the experiment is different from the theory. There is a
decrease in time taken for the suppository containing 0g of PEG 6000 to 3g of
PEG 6000 suppository and then drastically increases in the length of time taken
from 3g to 9g of PEG. This is considered that the experimental result is
deviated from the original theory as supposedly the 0g PEG suppository will
have the faster rate in melting process. This probably maybe caused by several
errors when we carried out the experiment. One of the errors is we could not
accurately observe and determine whether the suppository started to melt in the
37°C water bath with naked eyes. When we took out the beaker from the water
bath, we required looking carefully the small particles dispersed in the water
to indicate that the suppository started to melt and then it took time and led
to the inaccuracy of time recording. In addition, the temperature of the water
in the beaker should be consistent measured to ensure the water temperature was
maintained at 37°C due to it was conducted in open air condition. Moreover, the
rate of melting process of the suppository maybe affected by the inappropriate size
of beaker (50mL beaker was used) being used that hindered an even distribution
of heat throughout the suppositories. It is suggested that using test tube
instead of using the beaker so that even distribution of the heat can be
contributed to the suppositories.
4.
Describe function(s) of each ingredients used in the suppository formulation.
The ingredients used in preparing paracetamol
suppository formulation included polyethylene glycol (PEG) 1000, polyethylene
glycol (PEG) 6000, paracetamol, distilled water and liquid paraffin.
Polyethylene glycol (PEG) polymer is
commonly used as suppositories base due to it characteristics like
water-miscible and chemical stability. The characteristics of hardness, melting
point and dissolution time of PEG mainly depends on different molecular weight
of PEG types. PEG 1000 is a low molecular weight PEG, and when it is used
alone, it produced a soft suppository that is theoretically, would dissolute
and release the loaded drug fast. Whereas PEG 6000 is a high molecular weight
PEG, and when it is used alone, would produce hard and brittle suppositories,
thus causing a low release rate of drug. A good quality of base could be established when there is a combination of
right ratio between high molecular weight and low or medium molecular weight
PEG. The satisfactory or desirable hardness, melting point and dissolution
could then be achieved by altering the ratio of PEGs combined.
Besides,
paracetamol is the active ingredient for suppositories. It is used for
analgesic and anti-pyretic purposes. Paracetamol is dissolved in distilled
water as a solvent to form paracetamol solution.
Distilled
water is used as a solvent to incorporate a water-soluble substance in the
suppository base.
Whereas, liquid paraffin is used to
lubricate the mold before the solution filled in it. This is to ensure that the
suppositories does not stick to the mould and can be removed easily without the
cracking of suppositories.
Conclusion:
Calibrating suppositories mould with PEG before
preparing medicated suppositories is important as it ensures accurate dosing of
suppositories to be produced for the patients. Different compositions of PEG
base will affect the physical characteristics of the suppositories produced,
including their physical appearance, colour, hardness, greasiness and time
taken to start melting. When the highest
amount of PEG 6000 base is used to prepare the suppositories, the suppositories
produced are the hardest, smoothest, least greasy and are clear white in
colour. They are also most difficult to be melted as the longest time is taken
to let it start melting.
References: