Like all other dosage
forms, tablets and capsules are subjected to those pharmacopoeial standards
which deal with “added substances” with respect to their toxicity, interference
with analytical methods, etc. However, there are a number of procedures which
apply specifically to tablets and capsules, and which are designed, not only to
ensure that a tablet or a capsule exerts its full pharmacological actions, but
also to determine the uniformity of the physical properties of the official
tablet/capsule, irrespective of the manufacturer.
Such standards are
found in the British Pharmacopoeia and United Pharmacopoeia and include,
uniformity of diameter, uniformity of weight (mass), content of active
ingredient, uniformity of content, friability, disintegration and dissolution.
In addition there are a number of quality control procedures, which, though
widely applied, are not defined by the pharmacopoeias (non-pharmacopoeial
standards) such as thickness, and hardness.
The following
experiments demonstrate the application of a number of selected physical and
dosage performance tests on samples of commercially available tablets and
capsules. Students are required to refer to official pharmacopoeias for
detailed description of other tests not carried out in this practical session.
Experiment
1 : Physical appearance
Objective
To examine the shape, colour, diameter, thickness and other physical characteristics of tablet and capsule.
Apparatus & Materials
metre rule, tablet (Glument), capsule (Amolin)
Procedure
1. Any tablet and capsule are selected from the provided samples. For
the sample assigned, its shape, colour, diameter, thickness and other physical
characteristics are examined.
Results and Calculations
Tablet (Glument) : round, convex surface, white, opaque, 1 cm diameter,
0.4 cm thickness, odourless
Capsule (Amolin) : cylinder, red cap, yellow body, 0.5 cm diameter, 1.7
cm height, odourless, hard gelatin shell
A capsule is a solid dosage form in which the
drug is enclosed in a hard or soft soluble container, usually of a form of
gelatin. The two main types of capsules are hard-shelled capsules and
soft-shelled capsules. Hard-shelled capsules are typically made using gelatin and
contain dry, powdered ingredients or miniature pellets made by e.g. processes
of extrusion or spheronisation. These are made in two halves: a lower-diameter
"body" that is filled and then sealed using a higher-diameter
"cap". Soft-shelled capsules, primarily used for oils and for active
ingredients that are dissolved or suspended in oil.
Tablets and capsules may vary in shape,
colour, diameter, thickness and other physical characteristics, depending on
their intended use and method of manufacture.
In this experiment, we determined the
physical appearance of Glument (tablet) and Amolin (capsule). Glument has a round shape and convex surface.
It is white in colour and odourless is smell. Since the light cannot pass through
the tablet, it is opaque. The diameter and thickness of the tablet is 1 cm and
0.4 cm respectively. Amolin has a cylinder shape. This capsule made up of hard gelatin shell with a red cap and a
yellow body. The diameter and height of the capsule is 0.5 cm and 1.7 cm
respectively. It is odourless in smell.
Conclusion
The characteristics of the tablet (Glument) are round shape, convex
surface, white colour, opaque, 1 cm in diameter, 0.4 cm in thickness and
odourless. The characteristics of the capsule (Amolin) are cylinder shape, red
cap, yellow body, 0.5 cm in diameter, 1.7 cm in height, odourless and hard
gelatin shell.
www2.syphu.edu.cn/syzx/yjx/materials/jc_english/04.doc
https://en.wikipedia.org/wiki/Tablet_(pharmacy)
https://en.wikipedia.org/wiki/Capsule_(pharmacy)
Experiment 2 : Uniformity of diameter, thickness and hardness
Objective
To test the uniformity of diameter, thickness and
hardness of tablets.
Apparatus & Material
Procedure
Results
Apparatus & Material
10 tablets and Tablet Testing Instrument (PHARMATEST
PTB 311)
Procedure
1.
10 tablets are selected and the tests
for uniformity of diameter, thickness and
hardness using the Tablet Testing Instrument (PHARMATEST PTB 311) are carried
out.
2.
The deviation of individual unit from the mean diameter is calculated. It should not
exceed ± 5% for tablets with diameter of less than 12.5 and ± 3% for diameter
of 12.5 mm or more.
Results
Discussion
In experiment 2, a few
of pharmacopoeial and non-pharmacopoeial control tests are applied to the 10
tablets to be examined for the uniformity of diameter, thickness, and hardness. These tests are conducted to check if the
tablets have fulfilled the standard for the accepted specifications of the tablet.
The
diameter of the tablets is determined by the die and punches used in
compression. The deviation of the individual
unit from the mean diameter should not exceed ±
5% for tablets with a diameter of less
than 12.5 mm and ± 3% for the diameter of 12.5 mm or more, which follows the
standard that is found in the British Pharmacopoeia and United Pharmacopoeia. It
is one of the important features in quality testing of tablets and it is
important for the consistency of the appearance of the tablets. The uniform size
of tablets can avoid consumer confusion that different size of tablets
indicates different types of drug. Thus, the consumer
might overdose due to the excessive consumption of the same drug which can be
lethal. Uniformity of diameter
tests is applicable to all the uncoated and coated tablets except for the
enteric tablets, film-coated tablets, and
sugar-coated tablets. The tablets have the diameter more
than 12.5 mm and the mean of 12.81 mm. The deviation of the diameter is all in
the range of ± 3%. So, it can be
concluded that all ten of the tablets passed the test as none of the tablets is out of the range for the deviation
of the diameter of each tablet. The slight variations in diameter of the
tablets may be due to an uneven surface of punch and die.
The thickness of the
tablets is determined by the position of the punches in relation to each other
during compression. This is included in the number of quality control
procedures, which, though widely applied, are not defined by the pharmacopoeias (non-pharmacopoeial standards). Tablet
thickness is controlled to minimize the appearance problems, to assure the
tablets will fit into their designated container and to assure that the tablets
can be accurately counted as some filling equipment
depends on the uniformity of thickness of the tablets as a counting mechanism.
The tablets are slightly varied in thickness due to the difference in the granulation and pressure applied to the tablets, wear and tear on the length of punches as well as on the speed of tablet compression.
The hardness of the
tablets is tested by applying force required to break a tablet in a diametric
compression test. This is also included in the number of quality control
procedures, which, though widely applied, are not defined by the pharmacopoeias (non-pharmacopoeial standards).
The hardness test is important for the evaluation of the properties of tablets
because hardness is a significant physical parameter in the tablets control.
The tablets for oral consumption must be hard enough to be able to withstand mechanical
shocks of handling during its manufacture, packaging,
and transport. Besides, the tablets should be able to endure a reasonable amount of abuse when in the hands of
the consumer. Adequate tablet hardness and resistance to powdering and
friability are essential for consumer acceptance. Moreover, hardness may affect
tablet disintegration and makes the drug dissolution release rate to become
apparent.
Conclusion
Based on experiment 2,
all of the 10 tablets comply with the pharmacopeia standards proposed by having
uniform diameter, thickness
and hardness by the measurement from Tablet Testing Instrument.
References
1.
S. Ahmed, S. Ali, N. Haque, August 2001,
Evaluation of Acetaminophen tablets by control test, Pakistan journal of
pharmaceutical sciences [19 November 2016]
2.
T.
Bacalso, M. Baon, L. Cano, August 2014,
Tablet Hardness, Thickness and Diameter Test, https://prezi.com/v4lm0etmed6h/tablet-hardness-thickness-and-diameter-test/ [19 November 2016]
3.
WHO,
March 2011, REVISION OF MONOGRAPH ON TABLETS, Final text for addition to The
International Pharmacopoeia, http://www.who.int/medicines/publications/pharmacopoeia/Tabs-GeneralMono-rev-FINAL_31032011.pdf [19 November 2016]
Experiment 3 : Tablet Friability
Objective
To determine the tablet friability.
Apparatus & Material
Tablets, weighing machine, table abrasion, friability tester, brush
Procedure
1.
10
tablets are selected and be weighed.
2.
All
tablets are put into the drum of the table abrasion and friability tester. The
rate of rotation are set to 25 rpm, time to 10 minutes and the operation is
started.
3.
At
the end of the operation, all the tablets are removed and the freedom from dust
or powder (the brush is used) is ensured. The tablets are reweighing. The
percentage loss of weigh is determined.
4.
The
compressed tablet should not lose more than 1% of its weight.
Result
Discussion
By
definition, friability alone means fragile, easily damaged, easily reduced to
powder, easily shattered or crumbled. Thus, tablet friability can be defined as
the tendency of the tablet to undergo aberration, breakage and capping that is
caused by the mechanical shock especially during the manufacturing, packing and
transporting process. To ensure that the drug can be administered at right dose
and effectively, the tablets must be formulated to be able to withstand the
external and internal factors, so that the tablets would not be affected by the
mechanical shock too much. Thus, the mechanical strength of the tablets played
a vital role. External factor is usually caused by the poor condition of the
punches or worn at their surface edges, resulting in “whiskering” at the tablet
edge, which show higher friability values while for internal factor, it is
influenced by the moisture content of tablet granules. Besides, other than the
mechanical shock factor, poor formulation of the tablets such as poor tablet design
(too sharp edges), low moisture content and insufficient binder, which also
contribute to the internal factors can lead to the tablet friability.
Furthermore,
the tablets need to be hard enough to withstand the mechanical shock and
friable enough to be disintegrate in the gastrointestinal tract. Therefore, it
is also important that the hardness or mechanical strength of the formulated
tablets does not affect the ability of the drug to disintegrate inside the body
upon the oral admission. To evaluate the resistance of the tablets to the
abrasion and shock experienced during manufacturing, packing and transporting
process or to decide the suitability of the tablets for further processing such
as coating, friability test are performed. Friability test can be measured
through the percentage loss of the initial weight tablets. In this experiment, 10
tablets are chosen randomly and total weight is determined. Then, the tablets
are insert in the drum of tablet abrasion and friability tester. Friability
tester is used to stimulate the conditions that the products will be exposed
during the process of production. The rate of rotation is set at 25 rpm for 10
minutes. After that, the tablets are reweighing again to determine the
percentage loss of the initial weight tablets. The standard percentage loss of
the tablets should not exceed by 1%, the non-pharmacopeia standard. In this experiment, the percentage loss of
the 10 tablets is 0.69%, which does not exceed the 1%. This indicates that the
tablets are able to withstand the mechanical shock and abrasion during the
test.
Conclusion
The
percentage loss of the tablets is 0.69%, which does not exceed 1%. This
indicates that the tablets are able to withstand the mechanical shock and
abrasion and has a desired physical strength. Thus, the tablets are considered
to pass the friability test.
References
1.
Introduction
to Friability Testing: http://www.copleyscientific.com/home/pharmaceutical-testing/friability-testing/introduction-to-friability-testing
2.
Friability
Test:
3.
Tablet
Friability, Hardness and Dissolution:
Experiment 4 : Uniformity of weight of tablets and capsules
Objective
To determine the uniformity of weight of tablets and capsules.
Apparatus & Materials
20 tablets, 20 capsules, weighing balance
Procedure
Tablets
1. 20 tablets
previously selected at random are weighed. The average weight is determined.
2. Tablets are
weighed individually and the percentage deviation of its weight for each tablet
is determined from the average weight.
3. The deviation
of individual weight from the average weight should not exceed the limits given
below.
Average
weight of tablet
|
Deviation
(%)
|
Number
of tablets
|
Less
than 80 mg.
|
± 10.0
|
Minimum
18
|
± 20.0
|
Maximum 2
|
|
80 mg
to 250 mg
|
± 7.5
|
Minimum
18
|
± 15.0
|
Maximum
2
|
|
More
than 250 mg.
|
± 5.0
|
Minimum
18
|
± 10.0
|
Maximum
2
|
Capsules
1.
20 capsules are selected at
random.
2.
One capsule is weighed.
Capsule is opened and the contents are removed as completely as possible. The
emptied shells are weighed. The net weight of its contents is determined, that
is by subtracting the weight of the shells from the weight of the intact capsule.
3.
The procedure is repeated
with other 19 capsules.
4.
The average net weight is
determined from the sum of the individual net weights.
5.
The percentage deviation is
determined from the average net weight for each capsule. The deviation of
individual net weight should not exceed the limits given below:
Average
net weight of capsule
|
Deviation
(%)
|
Number
of tablets
|
Less
than 300 mg.
|
± 10.0
|
Minimum
18
|
± 20.0
|
Maximum
2
|
|
300 mg
or more
|
± 7.5
|
Minimum
18
|
± 15.0
|
Maximum
2
|
|
Results
and Calculations
Tablet
Total weight of
20 tablets: 11.4 g
Average weight
of one tablet: 570 mg / 0.57 g
Percentage of
deviation = Weight of individual tablet –
average weight of tablets
x 100%
Average
weight of tablets
Deviation of
5% = 0.57 g ± 5%
= 0.542 g to 0.599 g
Deviation of
10% = 0.57 g ± 10%
= 0.513 g to 0.627 g
Tablet
|
Weight
(g)
|
Percentage
deviation (%)
|
1
|
0.564
|
-1.05
|
2
|
0.583
|
+2.28
|
3
|
0.561
|
-1.58
|
4
|
0.573
|
+0.53
|
5
|
0.572
|
+0.35
|
6
|
0.580
|
+1.75
|
7
|
0.573
|
+0.53
|
8
|
0.571
|
+0.18
|
9
|
0.571
|
+0.18
|
10
|
0.572
|
+0.35
|
11
|
0.584
|
+2.46
|
12
|
0.561
|
-1.58
|
13
|
0.581
|
+1.93
|
14
|
0.590
|
+3.51
|
15
|
0.562
|
-1.40
|
16
|
0.582
|
+2.11
|
17
|
0.560
|
-1.75
|
18
|
0.554
|
-2.81
|
19
|
0.573
|
+0.53
|
20
|
0.570
|
0
|
Capsule
Total net weight
of capsule: 10.2855 g
Average net content
of the capsule: 514.3 mg / 0.5143 g
Percentage
of deviation: Net content of capsule - average net
content of capsule
x 100%
Average
net content of capsule
Deviation of 10% = 0.5143 g ± 7.5%
= 0.4757 g to 0.5529 g
Deviation of 20% = 0.5143 g ± 15%
=
0.4372 g to 0.5914 g
Capsule
|
Weight of intact capsule (g)
|
Weight of emptied shell (g)
|
Net weight of content (g)
|
Deviation (%)
|
1
|
0.6023
|
0.096
|
0.5063
|
-1.56
|
2
|
0.6143
|
0.096
|
0.5183
|
+0.78
|
3
|
0.6032
|
0.096
|
0.5072
|
-1.38
|
4
|
0.6011
|
0.096
|
0.5051
|
-1.79
|
5
|
0.6002
|
0.094
|
0.5062
|
-1.57
|
6
|
0.6015
|
0.104
|
0.4975
|
-3.27
|
7
|
0.6037
|
0.093
|
0.5107
|
-0.70
|
8
|
0.5958
|
0.085
|
0.5108
|
-0.68
|
9
|
0.6053
|
0.096
|
0.5093
|
-0.97
|
10
|
0.5990
|
0.091
|
0.5080
|
-1.22
|
11
|
0.5954
|
0.095
|
0.5004
|
-2.70
|
12
|
0.6038
|
0.095
|
0.5088
|
-1.07
|
13
|
0.6147
|
0.094
|
0.5207
|
+1.24
|
14
|
0.6033
|
0.093
|
0.5103
|
-0.78
|
15
|
0.6002
|
0.095
|
0.5052
|
-1.77
|
16
|
0.6055
|
0.097
|
0.5085
|
-1.13
|
17
|
0.6103
|
0.094
|
0.5163
|
+0.39
|
18
|
0.6043
|
0.096
|
0.5083
|
-1.17
|
19
|
0.6041
|
0.095
|
0.5091
|
-1.01
|
20
|
0.6153
|
0.095
|
0.5203
|
+1.17
|
Discussion
Test of the uniformity of weight of tablets and
capsules is carried out to ensure the consistency of dosage unit. The term “dosage
units” are defined as dosage forms containing a single dose or a part of a dose
of drug substance in each unit. The uniformity of dosage units specification is
not intended to apply to suspensions, emulsions, or gels in unit-dose
containers intended for topical administration. The term “uniformity of dosage
unit” is defined as the degree of uniformity in the amount of the drug
substance among dosage units. Uniformity of weight of drug is important to
ensure the even distribution of ingredients in the drug. Uneven distribution
may alter the dose in each individual drug and cause problems such as unable to
reach the therapeutic range or reach the toxic range. To ensure the consistency
of dosage units, each unit in a batch should have a drug substance content
within a narrow range around the label claim.
In this experiment, we measured the weight of 20
tablets of and 20 capsules of. Then, we calculated the deviation percentage of
each tablet and capsule. By doing this, we are able to determine the uniformity
of weight of tablets and capsules.
According to the result obtained, the tablets used
has an average weight of 570.0 mg and it is in the category of ‘250 mg and
more’. All the 20 tablets did not exceed the limit of + 5.0%. Therefore all of
the 20 tablets pass the test and consider as a successful badge of tablets. For
the capsules, all 20 capsules have average net weight of 514.3 mg. Therefore it
is the ‘300 mg and above’ category. All the capsules have the percentage of
deviation within the + 7.5%. Therefore all of the 20 capsules pass the test and
consider as a successful badge of capsules.
Conclusion
All the 20 tablets did not exceed the limit of + 5.0%. All the capsules have the percentage of deviation within the + 7.5%. Therefore all of the 20 tablets and 20 capsules pass the test and consider as a successful badge of capsules.
References
Apparatus & Material
20 Ibuprofen tablets, weighing balance, 20ml chloroform water, beaker, filter paper, filter funnel, conical flask, hair dryer, ethanol, phenolphthalein solution, 0.1 M sodium hydroxide, burette, retort stand with clamp.
Procedure
1.
20
Ibuprofen Tablets previously selected at random were weighed and powdered.
2.
A
quantity of powder containing 0.5g ibuprofen was extracted with 20 ml
chloroform for 15 minutes and it was filtered through a filter paper.
3.
The
residue was washed with 3·10mL chloroform and gently evaporated the combined
filtrate just to dryness with a hair dryer. The residue was dissolved in 100 mL
with ethanol (96%) previously neutralized to phenolphthalein solution.
4.
The
solution was titrated with 0.1M sodium hydroxide to end point with phenolphthalein
solution as the indicator. When the solution turned from colourless to pink
colour, the reading of the volume of sodium hydroxide was recorded.
5.
The
content of ibuprofen was calculated whereby 0.1M sodium hydroxide is equivalent
to 0.02063g of C13H18O2.
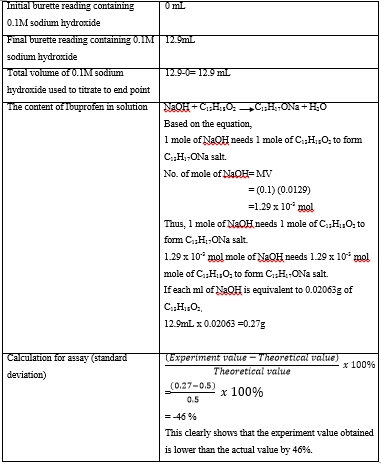
Results
One
tablet consists of 400mg ibuprofen
20
tablets = 400mg x 20
= 8000mg
=8g of ibuprofen
The
weight of 20 Ibuprofen Tablets = 10.24g
10.24g
of ibuprofen tablets powders contains 8g of ibuprofen
0.64g
of ibuprofen tablets powders contains 0.5mg of ibuprofen
Hence,
0.64g of ibuprofen tablets powders were weighed by an electronic balance and
dissolved in 20mL of chloroform.
The
calculation of the content of ibuprofen after titration
Discussion
Based on the
experiment, the experimental value of the content of ibuprofen in solution
obtained is 0.27g which is a large different compared to the theoretical value
of 0.5g if ibuprofen in 20 tablets. It
is obviously displayed that there is a difference of 46 % in percentage of
standard deviation between the values. The result is not satisfied as the
difference almost near to 50%. There may be several errors occurred during the
experiment was carried out that led to failure to get the satisfied result.
To begin with, there is
a possibility of the incomplete filtered of the mixture of the ibuprofen
tablets powders and the chloroform. The process of the mixture to be filtered
through the filter paper and filter funnel was very slow and the filter paper
has a coarse surface. Most possibly that the ibuprofen not dissolved in the
chloroform completely and remained on the filter paper due to the large
particle size of the ibuprofen. Therefore, the amount of the ibuprofen was
slightly reduced. This error can be overcome by filtering the mixture with sintered
glass crucible as it will be more effective and accelerate the process of
filtration.
In addition, one of the
errors is perhaps the active ingredient of the ibuprofen for this experiment
has been expired. Its efficacy and stability were reduced due to the
decomposition of the active ingredient. Thus, unexpired ibuprofen tablets need
to be confirmed before used to minimize the inaccuracy of the result.
Moreover, the filtrate
was not dry completely before the adding of sodium hydroxide process. We should
dry the filtrate with the hair-dryer for a longer period to ensure that all the
filtrate has been evaporated thoroughly. There is also possible that the
apparatus were contaminated from previous experiment. So, we need to clean the
apparatus and check whether they are totally ready to be used.
Questions
- What are
the objectives of the tests for uniformity of diameter and uniformity of
content ?
The main
objective of the test for uniformity is to guarantee that the tablets manufactured
are consistent in size and shape so that the efficacy and quality and be
maintained. It will also enhance the patient compliance as the appearance of
the tablets products easily to be recognised and prevent from being doubted by
patient for the dosage of the medication. Meanwhile, the test for uniformity of
the content is important to ensure that the content of active ingredients and
excipients in each of the tablets are similar. It is due to non-uniformity of
the tablets content will lead to overdose issues and may cause serious adverse
effects in patients.
- State the
types of tablets and capsules that must be tested for for uniformity of
diameter and uniformity of content.
All the uncoated and coated
tablets must be tested for the uniformity of diameter except for enteric
tablets, sugar- coated tablets and film-coated tablets due to tablet coating
affects the mass variation and diameter of the tablets. On the other hand,
uniformity of content tests are applicable for all tablets including
effervescent tablets, uncoated tablets and coated tablets and also all capsules
for example soft and hard capsules.
- Give
reasons for the non-compliance to test for uniformity of weight.
One of the
reasons is uneven feeding of the granules into the die of the tableting machine
as some of the granules will still remain in the die. Moreover, the non-uniform
movement of the lower punch lead to the variation in capacity of die space and
produce inconsistent weight of tablets.
References
1. http://apps.who.int/medicines/publications/pharmacopoeia/Tabs-GeneralMono-rev-FINAL_31032011.pdf
2. http://nsdl.niscair.res.in/jspui/bitstream/123456789/315/1/Tablet%20Technology%20Edited.pdf
Conclusion
Based on the experiment, the experimental value of the content of ibuprofen in solution obtained is 0.27g which is a large different compared to the theoretical value of 0.5g if ibuprofen in 20 tablets. There is a difference of 46 % in percentage of standard deviation between the values. References
1. http://apps.who.int/medicines/publications/pharmacopoeia/Tabs-GeneralMono-rev-FINAL_31032011.pdf



















No comments:
Post a Comment